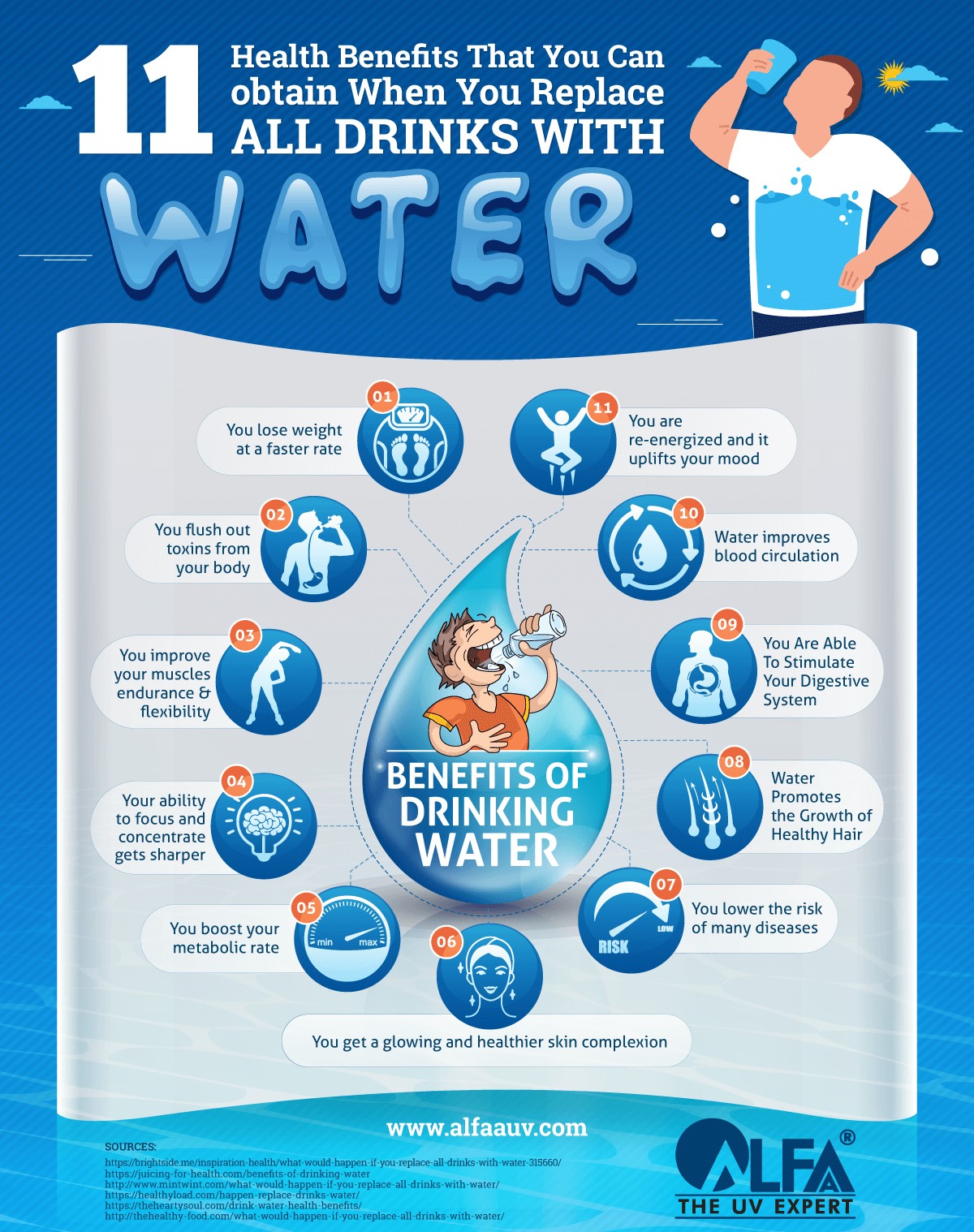
Properties Of Water Brochure
Properties Of Water Brochure - Explain why water is an excellent solvent; The hydrogen atoms bond to the oxygen atom through covalent bonds by sharing pairs of electrons. Water makes up about 71% of the earth's surface and 97% of that is found in oceans. The properties of water there are several properties of water which make it essential to living things. Why does water expand when it. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical formula h 2o. What are the physical and chemical properties of water that make it so unique and necessary for living things? Water is a colorless, odorless and tasteless liquid. Including solvation, cohesion versus adhesion, viscosity, buoyancy and thermal conductivity (water has a particularly high specific heat. Water really molecules stick together. When you look at water, taste and smell it—well, what could be more boring?. Water is everywhere, from huge oceans to invisible water molecules making up water vapor in the air. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical formula h 2o. Explain why water is an excellent solvent; The water molecule is comprised of two hydrogen atoms and one oxygen atom. Of course you can see and feel the physical properties of water, but. Why does water expand when it. What are the physical and chemical properties of water that make it so unique and necessary for living things? Static properties the chemical formula of water is h 2o. Water is a colorless, odorless and tasteless liquid. Of course you can see and feel the physical properties of water, but. The paper presents a comprehensive overview of the physical and chemical properties of water, emphasizing its critical role in the environment and its importance for life. These properties include its structure and its ability to act as a solvent for almost any. The properties of water “from. Water is a small solvent, occupying about 0.03 nm3 per molecule in the liquid state at room temperature and pressure, yet it is highly cohesive because of the strong intermolecular. Water really molecules stick together. Boiling point is defined as the temperature at which the vapour pressure of water becomes equal to. Including solvation, cohesion versus adhesion, viscosity, buoyancy and. Water is a small solvent, occupying about 0.03 nm3 per molecule in the liquid state at room temperature and pressure, yet it is highly cohesive because of the strong intermolecular. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical formula h 2o. Due to its shape and make up,. A water molecule is made of two. Explain why water is an excellent solvent; Water is a small solvent, occupying about 0.03 nm3 per molecule in the liquid state at room temperature and pressure, yet it is highly cohesive because of the strong intermolecular. The properties of water can best be understood by considering the structure and bonding of the. Static properties the chemical formula of water is h 2o. Water is a small solvent, occupying about 0.03 nm3 per molecule in the liquid state at room temperature and pressure, yet it is highly cohesive because of the strong intermolecular. The hydrogen atoms bond to the oxygen atom through covalent bonds by sharing pairs of electrons. The water molecule is. Including solvation, cohesion versus adhesion, viscosity, buoyancy and thermal conductivity (water has a particularly high specific heat. Explain why water is an excellent solvent; The unique properties of water are crucial for sustaining life on earth. Provide examples of water’s cohesive and adhesive properties; The arrangement of atoms in a water molecule, shown in figure 1.2,. The hydrogen atoms bond to the oxygen atom through covalent bonds by sharing pairs of electrons. Water makes up about 71% of the earth's surface and 97% of that is found in oceans. Water is a colorless, odorless and tasteless liquid. Describe the properties of water that are critical to maintaining life; The water molecule is comprised of two hydrogen. It has the formula h2o. Why does water expand when it. Water is everywhere, from huge oceans to invisible water molecules making up water vapor in the air. The hydrogen atoms bond to the oxygen atom through covalent bonds by sharing pairs of electrons. The unique properties of water are crucial for sustaining life on earth. States of matter, polarity, cohesion, adhesion, and its role as a universal solvent. Describe the properties of water that are critical to maintaining life; The water molecule is comprised of two hydrogen atoms and one oxygen atom. Water makes up about 71% of the earth's surface and 97% of that is found in oceans. Water really molecules stick together. The arrangement of atoms in a water molecule, shown in figure 1.2,. Due to its shape and make up, water molecules are polar molecules. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical formula h 2o. What are the physical and chemical properties of water that make it so. Boiling point is defined as the temperature at which the vapour pressure of water becomes equal to. A water molecule is made of two. What are the physical and chemical properties of water that make it so unique and necessary for living things? The arrangement of atoms in a water molecule, shown in figure 1.2,. Of course you can see and feel the physical properties of water, but. Water is a small solvent, occupying about 0.03 nm3 per molecule in the liquid state at room temperature and pressure, yet it is highly cohesive because of the strong intermolecular. States of matter, polarity, cohesion, adhesion, and its role as a universal solvent. Including solvation, cohesion versus adhesion, viscosity, buoyancy and thermal conductivity (water has a particularly high specific heat. Explain why water is an excellent solvent; Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical formula h 2o. Water is a colorless, odorless and tasteless liquid. Why does water expand when it. Water is everywhere, from huge oceans to invisible water molecules making up water vapor in the air. Water makes up about 71% of the earth's surface and 97% of that is found in oceans. Describe the properties of water that are critical to maintaining life; The properties of water can best be understood by considering the structure and bonding of the water molecule:Water Pamphlet Transform Global Health
County Water System Vance County
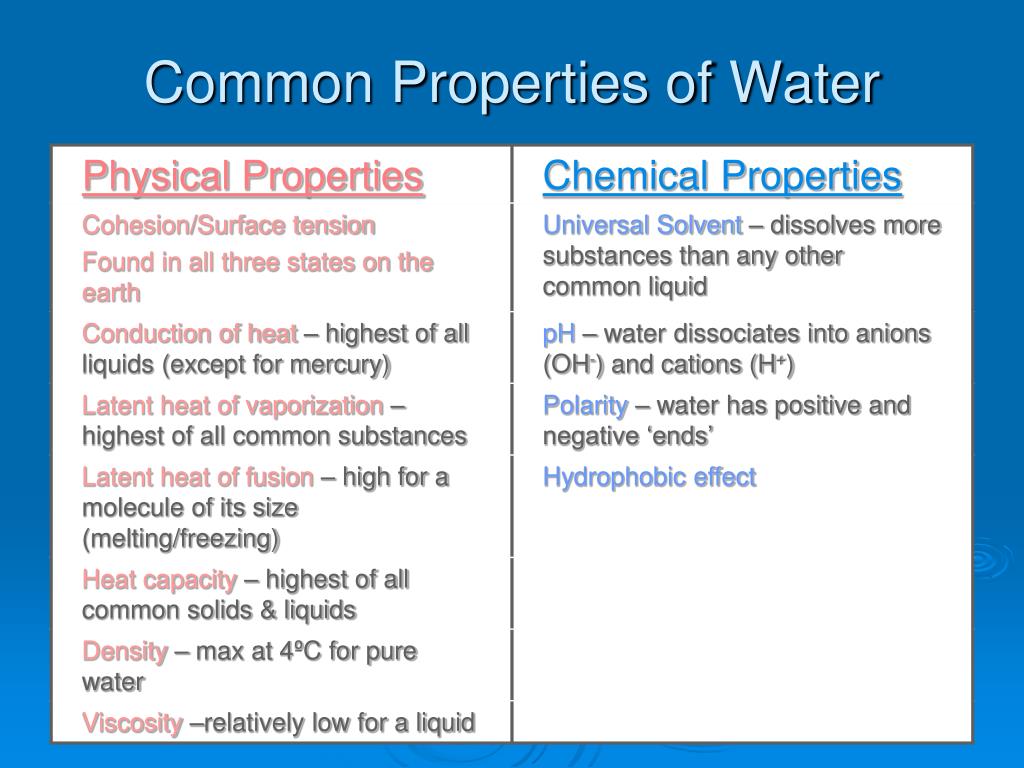
Physical Properties of Water
The world's purest water brochure design Behance
Properties of Water PDF Properties Of Water Buffer Solution
The healing and energetic properties of structured water… MOUNTAIN JUNKIE
The Structure and Unique Properties of Water Lesson 1.4 PDF
Properties Of Water
Properties of Water Lesson Plan Biology Teaching Ideas The Learning
Water Brochure Templates
These Properties Include Its Structure And Its Ability To Act As A Solvent For Almost Any.
It Has The Formula H2O.
The Water Molecule Is Comprised Of Two Hydrogen Atoms And One Oxygen Atom.
Water Is A Molecule Made Up Of Two Hydrogen Atoms And One Oxygen Atom.
Related Post: